Stratified Medicine Strategy Trials
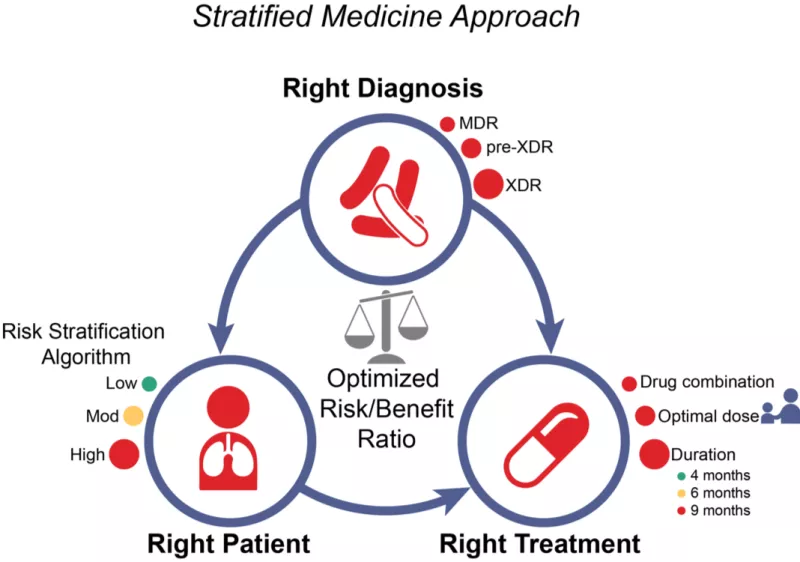
Current approaches to TB treatment are based on a ‘one-size-fits-all’ model of care. This approach is primarily program-centered, rather than patient-centered, and leads to under-treatment of patients with severe forms of disease and unnecessarily long, toxic treatment for a majority of patients in whom there is a less extensive disease burden. In routine care, this also leads to high rates of losses to follow-up. In clinical trials, ‘one-size-fits-all’ regimens have been consistently inadequate to cure the ‘hardest-to-treat’ TB patients. Drs. Rada Savic, Patrick Phillips and Payam Nahid and colleagues are leading the development of trials to test stratified medicine approaches to TB care, using pragmatic and readily available markers for risk stratification. Through an individual patient data meta-analyses of contemporary randomized phase 3 clinical trials in drug-susceptible TB plus additional cohort data from drug-resistant TB, routinely available baseline and on-treatment markers have been identified that can stratify burden of TB disease severity into easy-, moderate- and hard-to-treat phenotypes (Imperial M., et al., Nat Med 2018). With integration of newer, molecular-based diagnostic tests, the "Stratified PatiEnt-Centered Treatment Regimens for Active TB Trial" (SPECTRA TB) is under development and aims to match the right regimen with the right patient for the right duration, in place of the ‘one-size-fits-all’ paradigm used worldwide for decades. The overarching mission of the SPECTRA TB Strategy is minimizing toxicity, improving tolerability, and thereby significantly increasing treatment completion rates, while maintaining maximal cure rates for all patients.
PI: Rada Savic, PhD, Patrick Phillips, PhD and Payam Nahid, MD, MPH
---
Vietnam NTP/UCSF TB Research Collaboration (VNTP/UCSF)
Established through a 10-year CDC-funded contract, led by Dr. Payam Nahid and Viet Nguyen Nhung, the Vietnam National TB Programme and UCSF have establish a clinical trials unit in Hanoi, Vietnam. A total of 8 district health centers combined with the Hanoi Lung Hospital, recruit into TB therapeutics studies, and participate in biomarker and PK/PD substudies. Dr. Nahid is co-Chair of the ongoing Phase 3 clinical trial, TBTC Study 31 / ACTG A5349 evaluating high dose daily rifapentine for treatment shortening (Clinicaltrials.gov: NCT02410772), currently being conducted across TBTC and ACTG network sites.
PI: Payam Nahid, MD, MPH and Viet Nguyen Nhung, MD, PhD
---
DRAMATIC Phase 2 Duration Randomized MDR-TB Treatment Trial
An innovative Phase 2 study design (“duration-randomization”) to identify the shortest effective treatment duration. In this design, participants are randomized to four durations of treatment from the shortest to the longest likely effective duration. The results are then analyzed together to determine the optimal treatment duration. In the proposed multicenter, randomized, partially blinded, four-arm, phase 2 DRAMATIC Trial (Duration Randomized Anti-MDR-TB And Tailored Intervention Clinical Trial) we will examine the efficacy and safety of an all-oral regimen of bedaquiline, delamanid, levofloxacin, linezolid, and clofazimine given for 16, 24, 32 or 40 weeks. By modeling the results of the four durations together, the design achieves substantial statistical efficiency. The optimal treatment duration identified in this trial can then be validated in a larger prospective Phase 3 clinical trial.
Sponsor: NIH, NIAID
PI: Payam Nahid, MD, MPH and Robert Horsburgh MD, MUS
---
University of Zimbabwe-University of California, San Francisco Collaborative Research Programme (UZUCSF)
The University of Zimbabwe-University of California San Francisco Collaborative Research Program (UZ-UCSF) was established in 1994 to implement high quality science addressing HIV prevention and therapy; its aim is to control Zimbabwe’s HIV/AIDS epidemic and contribute to global policy as a center of excellence at the University of Zimbabwe College of Health Sciences (UZCHS) in collaboration with UCSF and its partner institutions. UZUCSF is the site of a well-established, large Clinical Trials Unit (CTU) with 4 Clinical Research Sites (CRSs), covering 6 distinct geographic locations within and surrounding Harare, Zimbabwe. Four Division of AIDS (DAIDS) Networks (HPTN, MTN, ACTG, IMPAACT), are included in the CTU's activities. As part of ACTG-enrollment activities, UZUCSF enrolls into CDC-TBTC Study 31, ACTG A5349.
Sponsor: NIH, NIAID
---
Opti-Q Phase II Trial of levofloxacin
Fluoroquinolones remain an essential part of MDR-TB treatment, but the optimal dose of fluoroquinolones as part of the regimen has not been defined. Opti-Q is a randomized, blinded, phase II trial in MDR-TB patients comparing across levofloxacin doses of 11, 14, 17 and 20 mg/kg/day, all within an optimized background regimen (see published protocol:https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-017-2292-x). The study aims to determine the levofloxacin AUC/MIC value that inhibits > 90% of organisms, the AUC/MIC that maximizes efficacy and the AUC that maximizes safety and tolerability in the context of an MDR-TB treatment regimen. 111 patients in sites in Peru and South Africa were randomized and final results are expected in 2018.
Sponsor: NIH Division of Microbiology and Infectious Diseases
UCSF PI: Patrick Phillips, PhD (PI: Bob Horsburgh, MD at Boston University, Boston)
---
Pan-African Consortium for the Evaluation of Antituberculosis Antibiotics (PanACEA)
The PanACEA consortium (www.panacea-tb.net) brings together scientists from 11 countries and 16 institutions in Africa, Asia and the US to conduct randomized clinical trials to evaluate new regimens for the treatment of TB. In partnership with pharmaceutical companies, PanACEA evaluates regimens including new chemical entities, repurposed drugs, and optimized doses of existing TB drugs. The mission of PanACEA is threefold: 1) To shorten and simplify treatment of uncomplicated pulmonary TB, 2) To increase the TB clinical trial capacity in Africa, and 3) To develop sustainable TB clinical trials network in Africa. UCSF leads the statistics and trial design expertise core for the consortium.
Funder: The European & Developing Countries Clinical Trials Partnership, EDCTP
UCSF PI: Patrick Phillips, PhD (PanACEA secretariat coordination is located at Radboudumc Nijmegen University)
---
Methodology to improve the design of TB clinical trials
Despite increased numbers of large late-phase clinical trials in recent years, we are still no closer to shortening treatment for drug-sensitive TB, although some progress has been made in MDR-TB. In the climate of dwindling funding for TB research and development, more efficient clinical trial designs can speed the evaluation of novel TB regimens. This leads to faster patient benefit by putting new regimens in the hands of TB physicians more quickly without compromising rigor in evaluating efficacy and safety. Activities in this program include work to improve how non-inferiority trials are designed and analysed, the implementation of adaptive clinical trials, the evaluation of surrogate and intermediate endpoints, novel designs to efficiently evaluate the optimal duration of a regimen alongside the evaluation of efficacy and safety, and more efficient methods of analysis to handle missing data in TB clinical trials.
---
Mechanism-Based Integrative Pharmacology Model for Tuberculosis
We use computational methods to study the dynamic interplay between disease progression, drug and biomarker response across relevant scales (molecule, cell, tissue, organ & whole body) in order to determine causal links underlying variability in (safety and efficacy) clinical outcomes. By integrating multi-scale, and multi-level clinical data, we aim to determine the right dose, right schedule and right treatment duration of various therapies, potentially bringing novel, precise and personalized treatment options to patients with unmet need more quickly.
Sponsor: Critical Path to TB Drug Regimens (CPTR)
PI: Rada Savic, PhD
---
Lesion-centric imaging and PK-PD of pyrazinamide for TB/HIV co-infection
To rationally design optimal drug regimens that will effectively prevent tuberculosis (TB) activation in HIV patient populations, new anti-TB drug regimens that have improved sterilizing properties are required. The proposed studies seek to provide pharmacokinetic and pharmacodynamic interpretation of the unique sterilizing effect of pyrazinamide. A better understanding of pyrazinamide's contribution to anti-TB therapy and its synergistic properties will inform the design and development of complementary drug combinations against TB-HIV co-infection.
Sponsor: Rutgers University
PI: Rada Savic, PhD
---
Novel Methods Of Pharmacologic Monitoring For Multidrug Resistant Tuberculosis Treatment In The Setting Of HIV Infection
A major barrier to routine pharmacologic monitoring within clinical trials and among high-risk patients (such as persons with concomitant MDR-TB and HIV) is that current methods to monitor medication exposure (i.e., plasma levels) require phlebotomy, a cold chain, and are generally not repeated frequently enough to characterize drug exposure over time. Moreover, high post-dose exposure in plasma after a directly-observed dose cannot confirm long-term adherence. Our goal in proposing this study is to determine whether an assessment of a panel of second-line TB drug concentrations in small hair samples, an easily accessible biomatrix, determined via liquid-chromatography /tandem mass-spectrometry will improve our ability to predict the risk of treatment failure, acquired drug resistance, or death in patients with MDR-TB, with a particular emphasis on those with HIV co-infection, in ongoing clinical trials of MDR-TB evaluating bedaquiline and delamanid - the first new drug classes created specifically to treat TB in over 50 years - and a South African Medical Research Council-supported phase III pragmatic randomized controlled trial examining the effectiveness of a novel 6-month, injectable-free MDR-TB regimen.
Sponsor: NIH, NIAID
PI: John Metcalfe, MD, PhD, MPH and Monica Gandhi, MD, MPH
---
Urban ARCH (3/5) Uganda Cohort TB Preventive Therapy For HIV-Infected Alcohol Users In Uganda: An Evaluation Of Safety Tolerability And Adherence
The Alcohol Drinkers' Exposure to Preventive Therapy for TB (ADEPT-TB) study is a continuation of the Uganda Alcohol Research Collaboration on HIV (ARCH) cohort of HIV-infected alcohol users in Uganda. Approximately one quarter of HIV- infected persons in sub-Saharan Africa (SSA) are heavy drinkers, but no studies have systematically assessed the safety of TB preventive therapy in alcohol users. Thus, the ADEPT-TB study aims to determine the safety and tolerability of TB preventive therapy for HIV-infected drinkers, and examine if the benefits in preventing TB outweigh the risks. Adherence to TB preventive therapy impacts the level of benefit and may also impact hepatotoxicity. Alcohol use is an established risk factor for decreased ART adherence and active TB treatment discontinuation, but it is not known whether HIV-infected drinkers on daily ART can be adherent to TB preventive therapy. The ADEPT-TB study will examine the safety and tolerability of, and adherence to, 6 months of daily INH (6H) and 3 months of daily INH plus rifampicin (3HR) in 380 randomized HIV-infected drinkers in Uganda.
Sponsor: NIH, NIAAA
PI: Judith Hahn, PhD
---
Options for Delivery of Short-Course Tuberculosis Preventive Therapy: The 3HP Options Trial
Tuberculosis (TB) remains the leading cause of death among people living with HIV (PLHIV) but can be prevented with medications, including a recently approved 12-dose regimen. This proposed studies will provide a definitive, comprehensive evaluation of three strategies – including offering patients a personal choice – for delivering this new treatment regimen to PLHIV in a high TB-burden, low-income setting. The results of this trial will inform policy and scale-up, with the ultimate aim of improving the health of PLHIV worldwide.
Sponsor: NIH, National Heart, Lung, and Blood Institute
PI: Adithya Cattamanchi, MD and David Wesley Dowdy, MD, PhD
---
Core I: Pharmacology Core
The UCSF-Gladstone CFAR Pharmacology Core has provided state of the art pharmacological tools for HIV-associated research for over 30 years, with the overarching goal of optimizing treatments for HIV- infected subjects. T the Core is the home-base for long-standing scientists and staff who support CFAR investigators through analytical assay development, pharmacological trial design, and sample and data analysis for preclinical and clinical pharmacokinetic (PK) and pharmacodynamic (PD) studies. In the last five years, the Pharmacology Core analyzed over 27,000 samples, contributed to 44 published manuscripts, provided mentoring and training to 24 faculty, fellows, and students, and supported 35 investigations led by 32 investigators. The Core responded to the changing needs of CFAR investigators by introducing 26 new assays and services and actively supported CFAR scientific priorities including treatment for HIV, TB, and malaria, particularly internationally, and inflammation and latency. In addition to strong support toward domestic CFAR initiatives, the Core is heavily integrated with CFAR sponsored international research including with programs in Kampala and Tororo, Uganda; Kisumu, Kenya; and Harare, Zimbabwe. The Core continuously evolves to maintain a broad array of cutting-edge pharmacological assays including methods to quantitate the most current drugs used in HIV patients based on plasma, intracellular, and tissue-based assays. The Core focuses on three complementary efforts that together provide added value to CFAR-affiliated investigators– 1) Supporting CFAR investigators with specialized pharmacological methodologies and expertise in study design or data analysis customized to their needs; 2) Advancing synergy by supporting translational research relevant to CFAR Scientific Priorities; and 3) Providing education and training in pharmacological analytical methods, study design and analysis for next generation translational scientists, both domestically and internationally.
Sponsor: NIH, National Institute of Allergy and Infectious Diseases
---
Simplified Isoniazid Preventive Therapy (SPIRIT) Strategy to Reduce TB Burden
Although prior research studies tell us that persons living with HIV at risk for TB can protect themselves for getting TB by taking the antibiotic isoniazid for 6 months, less than 1% of persons eligible receive this intervention. We found that the regional health managers responsible for the public health in their region are not knowledgeable about this intervention, have perceived concerns about putting it into operation and have challenges in overseeing their many clinics. This is an implementation research study where we study whether a multicomponent intervention (SPIRIT) that assists the District Health Officers can overcome these barriers. Testing an intervention that could improve worldwide delivery of isoniazid prevention for persons living with HIV in high TB burden countries could dramatically reduce global morbidity and mortality from TB.
Sponsor: NIH, National Institute of Allergy and Infectious Diseases
PI: Diane Havlir, MD
---
Safety and tolerability of new and repurposed drugs for MDR-TB treatment
Two new drugs, bedaquiline and delamanid, and two repurposed drugs, clofazimine and linezolid, were recently recommended for multidrug-resistant tuberculosis (MDR-TB) treatment. A growing body of data is emerging from clinical trials of novel MDR-TB regimens using these drugs, yet high-quality evidence on their safety and tolerability—informed by pharmacokinetics—is sorely lacking. We will leverage existing and forthcoming data to [1] examine the safety and tolerability of bedaquiline and delamanid within conventional MDR-TB regimens; [2] estimate the effect of linezolid-dose reduction strategies on the safety and tolerability of MDR-TB treatment and the pharmacokinetics of linezolid; and [3] estimate the effect of plasma drug exposure on the safety and tolerability of combinations of new and repurposed drugs in a Phase III trial.
Sponsor: NIH, National Institute of Allergy and Infectious Diseases
PI: Gustavo Velasquez, MD, MPH
---
UCSF-Gladstone Center for AIDS Research
The mission of the UCSF-Gladstone Center for AIDS Research (CFAR) is to promote multidisciplinary research at the intersection of the basic, clinical, and behavioral-epidemiological sciences with the goal of ending the global HIV epidemic. The program will provide administrative and scientific leadership through a proactive planning process that identifies the most important challenges emerging at the cutting edge of HIV research; Identify, mentor, and support a highly skilled, diverse, and thoughtful next generation of HIV investigators by providing a strong mentoring program unique to UCSF and through a California-funded Health Disparities Core linked to CFAR; Conduct a dynamic pilot grants program to accelerate discovery; Maintain an outstanding set of scientific cores to extend the reach of Center investigators' research; Ensure our programs support major NIH-funded HIV/AIDS research programs and OAR priorities; Confront domestic prevention and treatment disparities through effective local collaborations; Direct CFAR's research and capacity building programs to international sites where the epidemic is hitting the hardest Engage the communities we serve through a set of novel alliances involving Project Inform, the Forum for Collaborative HIV Research, and UCSF's Science and Health Education Partnership; and Forge effective inter-CFAR collaborations to nucleate research teams across different disciplines and sites to address all dimensions of these identified challenges. CFAR brings value by creating and sustaining a true community of HIV/AIDS science.
Sponsor: NIH, National Institute of Allergy and Infectious Diseases
---
Pharmacokinetics and tolerability of adjunctive linezolid for the treatment of tuberculous meningitis
This open label randomized controlled trial will evaluate the pharmarcokinetics and tolerability of a 4-week course of adjunctive linezolid administered with high and standard dose rifampin early in the treatment of TBM in adults living with HIV in Uganda, which has a large burden of HIV/TB co-infection. The knowledge gained from this trial will be used to determine an optimal linezolid dose for a future efficacy trial of adjunctive linezolid to improve clinical outcomes in TBM.
Sponsor: Fogarty International Center
---
